JUVÉDERM® Dermal Fillers
Ask About Smoothing Away Wrinkles and Folds With JUVÉDERM® XC Today
JUVÉDERM®XC is the first and only dermal filler for facial wrinkles and folds FDA approved to last up to 1 year with initial optimal correction
Our Patient Results
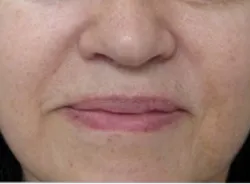
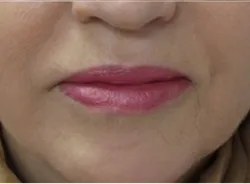
Lip Enhancement & Dermal Filler

Model was treated for parentheses lines (JUVEDERM ULTRA. 0.5 ml; JUVEDERM Ultra plus, 1.5. mL)marionette lines and corners lines (JUVEDErm Ultra 0.2 mL; JUVEDERM Ultra Plus, 0.2 mL) Total 3.2 mL.
JUVÉDERM® Contains lidocaine a common anesthetic to make the treatment process less painful.
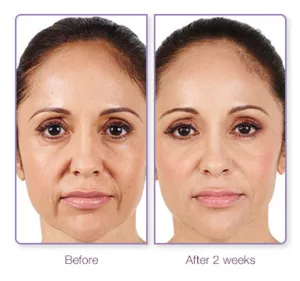
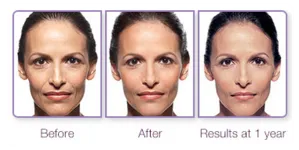
The before and after images above show the dramatic results 6 weeks and 1 year after treatment with JUVEDERM XC
Unretouched photos of paid JUVÉDERM® model treated with JUVÉDERM® Ultra XC for parentheses lines and marionette lines. A total of 3 syringes (1.0 mL each) was used. Photos taken before treatment, 6 weeks after treatment, and at 1 year after treatment. Individual results may vary.
Download the JUVÉDERM® XC Treatment Visualizer app* to see the difference for yourself.
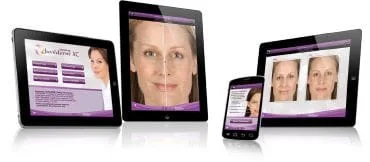
* The JUVÉDERM® XC Treatment Visualizer app is for illustrative and demonstrative purposes only. The results displayed may not reflect actual results. References: 1. JUVÉDERM® Ultra XC Patient Labeling, 2010. 2. JUVÉDERM® Ultra Plus XC Patient Labeling, 2010.
JUVÉDERM® XC Important Information

APPROVED USE
JUVÉDERM® XC injectable gel is for injection into areas of facial tissue where moderate to severe facial wrinkles and folds occur to temporarily add volume to the skin, especially around the nose and mouth.
IMPORTANT SAFETY INFORMATION
Are there any reasons why I should not receive JUVÉDERM® XC?
Do not use the product if you have severe allergies with a history of severe allergic reactions (anaphylaxis), or if you are allergic to lidocaine or the proteins (gram-positive bacterial proteins) used to make the hyaluronic acid (HA) in JUVÉDERM® XC.
What precautions should my doctor advise me about?
-
.
The safety of JUVÉDERM® XC injectable gel for use during pregnancy, in women who are breastfeeding, or in patients with very thin skin in the cheek area has not been studied
- The safety for use in patients under 18 years has not been studied
- The safety and effectiveness for treatment in areas other than facial wrinkles and folds (such as lips) have not been established
- The safety for use in patients with a history of excessive scarring or pigmentation disorders has not been studied and may result in additional scars or changes in pigmentation
- Tell your doctor if you are on therapy used to decrease the body’s immune response (immunosuppressive therapy). Use may result in an increased risk of infection
- Tell your doctor if you are using medications that can prolong bleeding, such as aspirin, ibuprofen, or other blood thinners; as with any injection, this may result in increased bruising or bleeding at the injection site
- If laser treatment, chemical peel, or any other procedure based on active dermal response is considered after treatment, or if the product is administered before the skin has healed completely, there is a possible risk of an inflammatory reaction at the treatment site
- As with all skin injection procedures there is a risk of infection
.
What are possible side effects?
The most commonly reported side effects for JUVÉDERM® XC injectable gel were temporary injection-site redness, swelling, pain/tenderness, firmness, lumps/bumps, bruising, itching, and discoloration. They were predominantly mild or moderate in nature and lasted 7 days or less.
To report a problem with JUVÉDERM® XC, please call Allergan Product Surveillance at 1-800-624-4261.
For more information, please see the
About Safety
page at
www.juvederm.com
or call the Allergan Medical Information line at 1-800-433-8871.
JUVÉDERM® XC injectable gel is available by prescription only.

©2014 Allergan, Inc., 2525 Dupont Drive, Irvine, CA 92612 ® and ™ marks owned by Allergan, Inc. JUVÉDERM® mark owned by Allergan Industrie, SAS. Android and Google Play are trademarks of Google Inc. App Store is a service mark of Apple Inc. www.juvederm.com APC13HK14 133621 SOLICITATION
If you no longer wish to receive emails from this sender about JUVÉDERM® XC products and services, please reply to this email and include "unsubscribe" in the subject line.




